The devastating effect of COVID-19 on the world created a global crisis and a great need for a vaccine to help people combat the disease. The seriousness of the situation led to government and health agencies across the planet coming together to figure out what to do about it.
In response, several companies began to work on vaccines with aid from government agencies. This extreme focus on getting something viable available for the millions of people who need it has been at the forefront of all coronavirus vaccine news. Here are what things look like now at the end of 2020.
What Are the Currently Approved Vaccines for COVID-19?
Currently, in coronavirus vaccine news, there are several vaccines in various stages of testing, but there are also some vaccines that have received approval in the United States as well as in other countries. No two vaccines are precisely the same, so each one that reaches approval can still work in a way altogether different from another approved vaccine.
So, who is making a coronavirus vaccine? Many companies have vaccines in the works. Nevertheless, only a few have made it to approval from a government agency.
Which Vaccines Are Approved in the United States?
One vaccine, the Pfizer-BioNTech COVID-19 Vaccine, is already approved and being rolled out around the nation. Another vaccine from Moderna, Inc. is also on the schedule for Emergency Use approval from the FDA. Novavax, who is making a coronavirus vaccine, is also in the works but has not garnered as much attention as the Pfizer and Moderna offerings.
Another one that saw a lot of press is the Oxford-AstraZeneca collaborative effort. Still, it is the Pfizer vaccine that is first to market with the Moderna vaccine potentially on the verge of gaining approval any day now.
The United States implemented a project called Operation Warp Speed with a stated goal of delivering 300 million doses to the public by January 2021. The timeline is aggressive and the sheer number of resources dedicated to the project is what is helping US companies accomplish the goal of developing these vaccines quickly.
Which Vaccines Are Approved Around the World?
The global situation when it comes to COVID-19 vaccines varies depending on the area in question. According to the World Health Organization, there are over 50 vaccines in various stages of development and at various trial levels. Some countries, such as Canada and the UK have authorized the use of the Pfizer-BioNTech vaccine with the possibility of the EU close behind in approving it as well.
Also, some countries, such as China and Russia, have been rolling out their own vaccines for some time now. These two countries specifically started making a coronavirus vaccine and distributing it before conducting any large clinical coronavirus vaccine trials. Those making a coronavirus vaccine in these countries include offerings from Sinovac Biotech, Gamaleya, CanSino Biologics, and Sinopharm. Some of these vaccines have seen use as early as the summer of 2020.

How Do All These COVID-19 Vaccines Work?
The most promising vaccines, such as those from Pfizer and Moderna, actually represent a breakthrough. These vaccines do not require people to have a live virus placed in their bodies. The mRNA vaccines they have developed are novel, although there are other mRNA drugs out there.
Basically, mRNA vaccines will attempt to trick the body into creating the proper immune response to COVID-19. There is far more to it than that, of course, as these mRNA vaccines are the culmination of years of study into how mRNA works. Nevertheless, the mRNA route is not the only route to a vaccine, but it is the fastest, which is why the first approved vaccines in the United States are mRNA vaccines.
As there are no 100% effective vaccines, that also applies to the COVID-19 vaccines. However, the vaccines already distributing and those close to approval report efficacy ratings of 90% or more, which is very good news.
Where Are Vaccinations Being Implemented?
As this is a current and ongoing issue facing the world, things are changing literally by the day when it comes to any coronavirus vaccine update. However, vaccination rollouts have already started in the United States with the Pfizer-BioNTech vaccine already being issued to people in the United States, Canada, and the UK. As noted, China and Russia have administered their respective vaccines for several months already.
The WHO has plans to move whichever viable vaccines pass their coronavirus vaccine trials to the rest of the globe as soon as possible. This all means that, with some caveats, the process of getting a vaccine to the populace is well underway in the US and abroad.
However, the nature of creating, storing, and distributing these vaccines can also create some bottlenecks that can mean the rollout of vaccines can stall or delay. The potential approval of the Moderna vaccine in the US can help alleviate some of these issues as it will create another avenue of vaccine distribution.

What Are Some of the COVID-19 Vaccine Concerns and Issues?
There are numerous concerns regarding COVID-19 vaccines, but many of them are the very same concerns that people have about all vaccines in general. Additionally, any coronavirus vaccine update can change from day to day when it comes to concerns and issues.
There is also a question priority, as in who will and should receive these vaccines first? The priority question is an important one as there are not an unlimited amount of vaccine doses, so what happens with the first lot matters.
Vaccine Availability
Health professionals and administrators want everyone who can receive a vaccine to receive one. However, at first, there simply will not be enough doses of any vaccine available to everybody. As time goes, the supplies will increase, but initially, these doses will only go to those who need them most. Also, the availability of doses for children may require more time and study.
Vaccine Distribution
Another challenge with these vaccines comes with how to distribute them properly. The Pfizer vaccine, which is the only one available in the US at the moment, has strict storage requirements that can make it difficult to move them and keep them viable before use. The federal government has a number of plans in place to make sure the doses reach the places they are supposed to through logistic coordination with several manufacturers and commercial organizations.
The CDC has the authority to work with states, territories, and reservations to make sure that vaccination plans are adequate in regards to receiving and distributing vaccines. In addition, numerous private companies and chains also play a role in working with the federal government to make sure the vaccines reach who they are supposed to.
Vaccine Priority
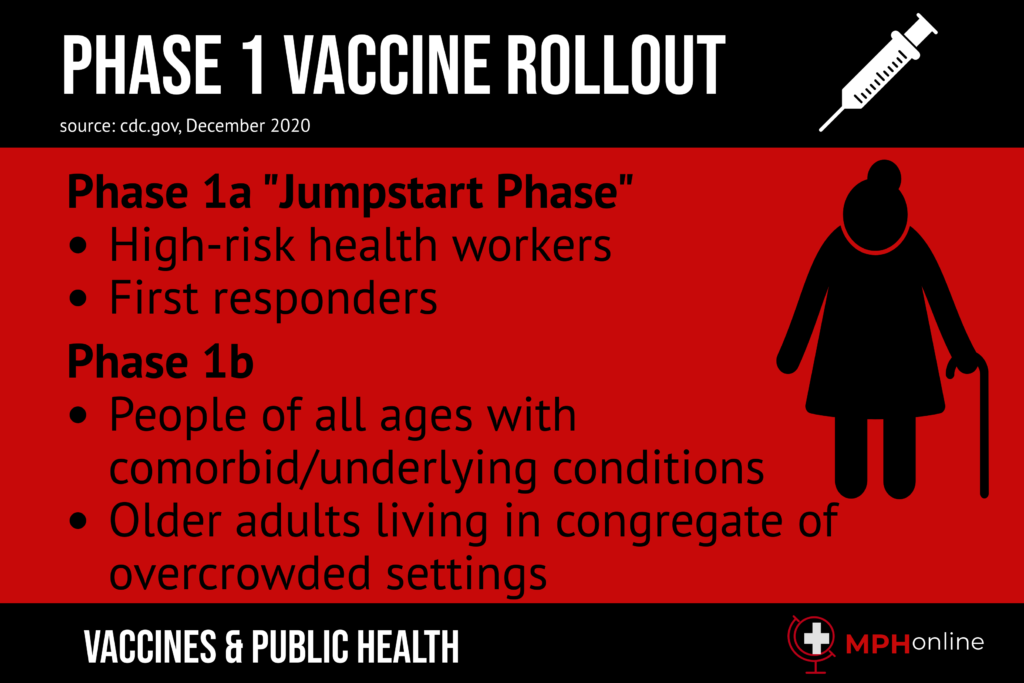
In most places, the question of who should receive the vaccine first has been left up to individual jurisdictions to decide. Generally, there is an almost universal agreement that the initial COVID-19 vaccination should go to those with the highest risk factors. This means the first doses will tend to go to healthcare workers and those who spend the most time exposed to the virus.
Another group with priority consists of long-term care residents. The CDC has established a priority list beyond that. Most of the decision of priority also has to do with the simple fact that there will not be enough doses to go around at the start, so there was a lot of discussion on how the country as a whole can benefit most based on who receives the vaccinations first.
The faster more vaccines receive approval, the more the availability of doses will increase. Also, since a lot of the priority discretion remains left up to individual jurisdictions, some people may receive priority in one state or locale than in others. For example, one state may place a priority on first responders and law enforcement while another may put the priority on other populations, such as teachers.
Vaccine Fears
One of the biggest categories of concern over COVID-19 vaccines has to do with fears stemming from misinformation, preconceived notions, a lack of research, and misunderstandings. Many of the questions and fears people have about these vaccines are easily answered and explained with a search.
Every major healthcare organization contains information, FAQs, and other data that can answer veritably any question or concern someone may have. Making a coronavirus vaccine requires a lot of time, effort, attention to detail, and testing. While there is a possibility of side effects for a minority of the population, this is no different from any vaccination or medication.
Nevertheless, several organizations are watching these vaccines very closely and will report anything concerning them immediately, so people do not have to fear being left in the dark should anything develop concerning one of the vaccines.
It is easy to see how vaccines in general encompass a tremendous number of industries. From start to finish, the creation, distribution, study of, and social health implications of any single vaccine creates an incredible number of career opportunities.
While the coronavirus pandemic has created a lot of negative effects around the world, it has also raised interest in how these things work and are studied. The more knowledgeable the populace, the more people will enter this field to raise awareness so that people can embrace what science offers.
Related:
- Best Practices During a Pandemic
- A Comprehensive Pandemic Survival Kit List
- How to Survive a Pandemic
- Coronavirus and Public Health
- What’s the Difference Between a Virus and a Syndrome
- What’s the Difference Between an Epidemic and a Pandemic
- Surfaces and Covid-19: A Public Health Perspective
- Fast-Tracking Coronavirus Vaccines: The Benefits and Risks